Amagat's Law:
Volume of an ideal gas mixture is equal to the sum of the partial volumes
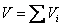 |
(34.13) |
V = total volume of the mixture
Vi = partial volume of the species i
= volume of the species if it existed alone in the given temperature T and pressure P
For an ideal gas
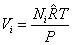 |
(34.14) |
Amagat's Law
or
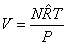 |
(34.15) |
The volume fraction of species i
or,
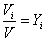 |
(34.16) |
Volume fraction = Mole fraction
Mass based analysis is known as gravimetric analysis
Mole based analysis is known as molar analysis |