Thermodynamic Definition of Work
In thermodynamics, work done by a system on its surroundings during a process is defined as that interaction whose sole effect, external to the system, could be reduced as the raising of a mass through a distance against gravitational force.
Let us consider the raising of mass m from an initial elevation z1 to final elevation z2 against gravitational force. To raise this mass, the force acting on the mass is given by F = mg . The work done on the body is W = mg( z2 - z1 )
- An external agency is needed to act on the system
- It can be seen that expansion of the gas gets reduced to raising a mass against gravitational force (Figure 4.2)
| dW = F dX = P A dX = P dV |
|
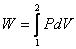 |
|
Figure 4.2
During this expansion process, the external pressure is always infinitesimally smaller than the gas pressure.
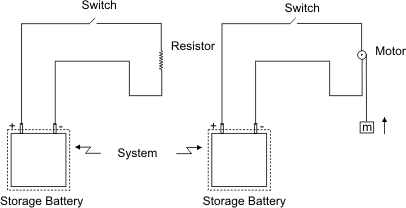
Figure 4.3
Compare two systems shown in the figure 4.3. Let the resistor be replaced by a motor drawing the same amount of current as the resistor. The motor can wind a string and thereby raise the mass which is suspended. As far as the battery is concerned, the situations are identical. So, according to thermodynamic definition of work, the interaction of a battery with a resistor is called work. By manipulating the environment, that is external to the battery (system), the effect can be reduced to raising of a mass against the gravitational force and that is the only effect on the surroundings. We can see that the thermodynamic definition of work is more general than that used in mechanics.
|