Situation in which W ≠ P dV
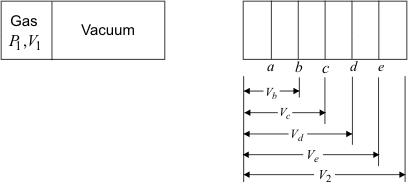
Figure 4.4
- Let the initial volume be V1 and pressure P1
- Let the final volume be V2 and pressure P2
What should be the work done in this case? Is it equal to ∫P dV ?
∫P dV = area under the curve indicating the process on P-V diagram.
The expansion process may be carried out in steps as shown in figure 4.4. It is possible to draw a smooth curve passing through the points 1bcde2 . Does the area under the curve (figure 4.5) represent work done by the system? The answer is no, because the process is not reversible. The expansion of the gas is not restrained by an equal and opposing force at the moving boundary.
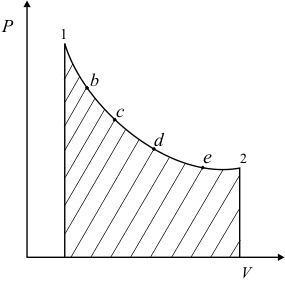
Figure 4.5
No external force has moved through any distance in this case, the work done is zero. Therefore, we observe that
| W = ∫ P dV only for reversible process |
|
| W ≠ ∫ P dV for an irreversible process |
|
Another exceptional situation !
- The piston is held rigid using latches ! (Figure 4.6)
- dV = 0
- Work done on the gas is equal to the decrease in the potential energy of mass m
- A situation where dV = 0 and yet dW is not zero
- such work can be done in one direction only. Work is done on the system by the surroundings
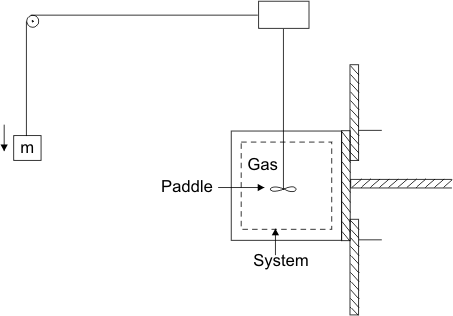
Figure 4.6
|