Heat
Heat is energy transfer which occurs by virtue of temperature difference across the boundary. Heat like work, is energy in transit. It can be identified only at the boundary of the system. Heat is not stored in the body but energy is stored in the body. Heat like work, is not a property of the system and hence its differential is not exact. Heat and work are two different ways of transferring energy across the boundary of the system.
The displacement work is given by (figure 4.7)
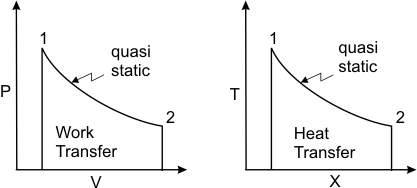
Figure 4.7 It is valid for a quasi-static or reversible process. The work transfer is equal to the integral of the product of the intensive property P and the differential change in the extensive property, dV .
Just like displacement work, the heat transfer can also be written as
The quantity dQ is an inexact differential.
X is an extensive property and dX is an exact differential. The extensive property is yet to be defined. We shall see later that X is nothing but the entropy, S of a system.
It is possible to write
or,
where 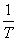 is integrating factor. is integrating factor.
|