Analysis of Closed System
Let us consider a system that refers to a definite quantity of matter which remains constant while the system undergoes a change of state. We shall discuss the following elementary processes involving the closed systems.
Constant Volume Process
Our system is a gas confined in a rigid container of volume V (Refer to Figure 6.4)
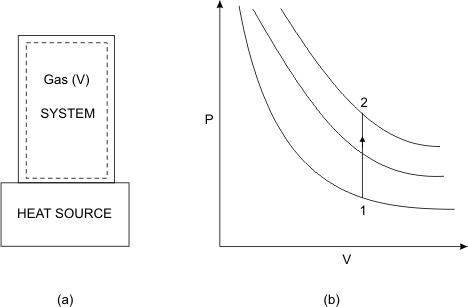
Figure 6.4
- Let the system be brought into contact with a heat source.
- The energy is exchanged reversibly. The expansion work done (PdV) by the system is zero.
- Applying the first law of thermodynamics, we get
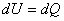 |
(6.12) |
or,
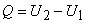 |
(6.13) |
- Hence the heat interaction is equal to the change in the internal energy of the system.
Constant Volume Adiabatic Process
Refer to Figure 6.5 change in state of the system is brought about by performing paddle wheel work on the system.
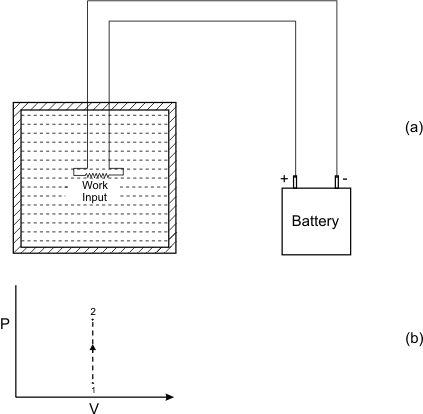
Figure 6.5
The process is irreversible. However, the first law gives
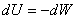 |
(6.14) |
or
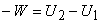 |
(6.14) |
Interaction of heat and irreversible work with the system is same in nature. (-W) represents the work done on the system by the surroundings
|