Specific Heat at Constant Volume
By definition it is the amount of energy required to change the temperature of a unit mass of the substance by one degree.
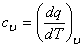 |
(6.16) |
While the volume is held constant. For a constant volume process, first law of thermodynamics gives
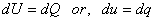 |
(6.17) |
Therefore,
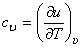 |
(6.18) |
Where 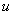 is the specific internal energy of the system. If is the specific internal energy of the system. If 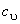 varies with temperature, one can use mean specific heat at constant volume varies with temperature, one can use mean specific heat at constant volume
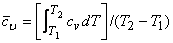 |
(6.19) |
The total quantity of energy transferred during a constant volume process when the system temperature changes from 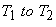
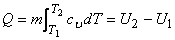 |
(6.20) |
The unit for 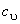 is kJ/kgK. The unit of molar specific heat is kJ/kmolK. is kJ/kgK. The unit of molar specific heat is kJ/kmolK.
|