

Click to view topics/project descriptions
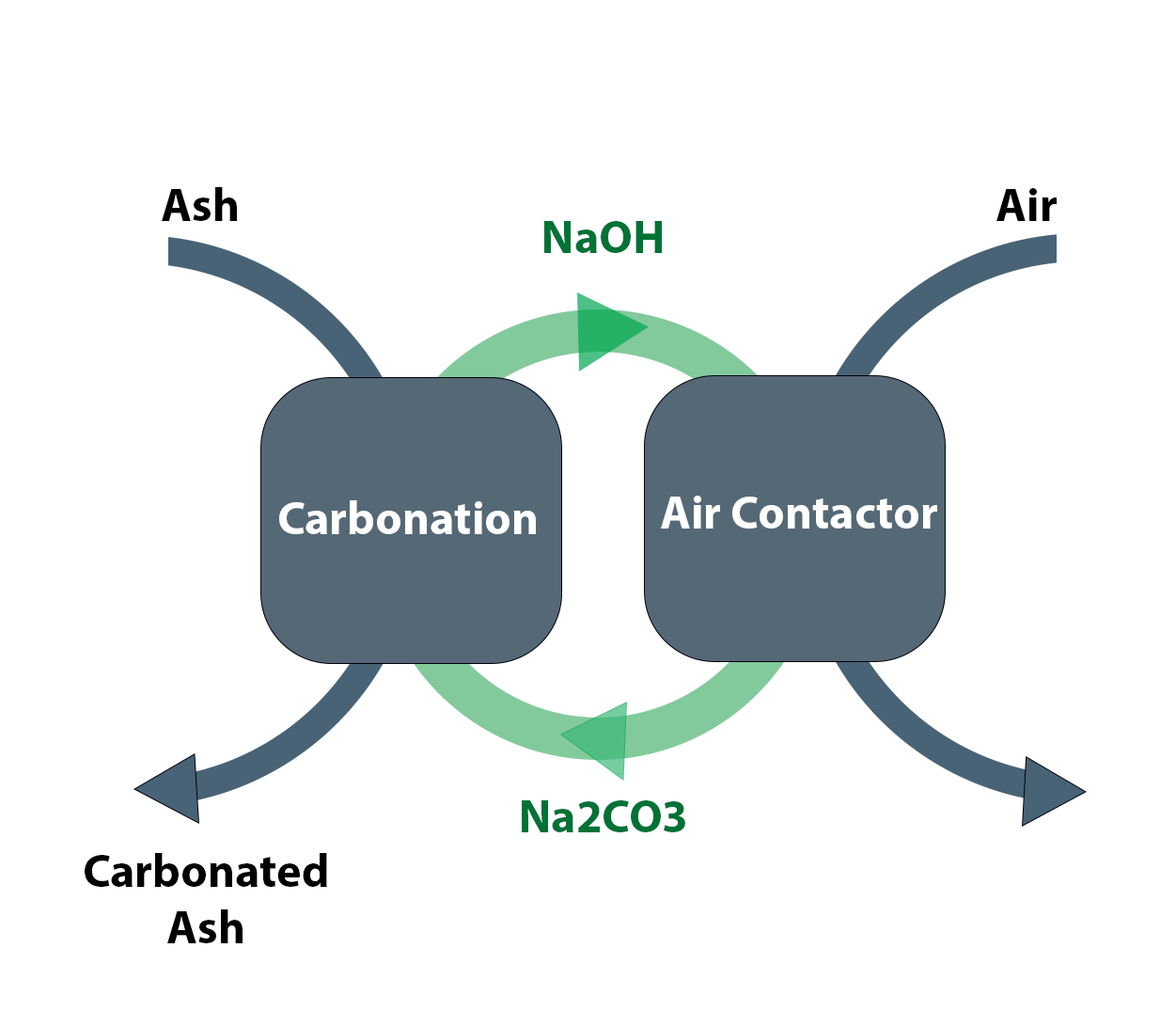
Fig. Schematic showing use of indusrial wastes and natural minerals for carbon dioxide capture & sequestration.
In the carbonation step, alkaline hydroxide solution is produced by carbonating CaO-rich solids in sodium carbonate solution. This reaction is similar to age-old causticizing reaction in Kraft's processes. The main challenge is in using complex feedstocks made up of silicates and aluminates instead of calcium oxide.
In the absorber part where CO2 is captured from a point of source or directly from air, hydroxide solution is used as a solvent to scrub carbon dioxide. This step regenerates the alkali carbonate solution used in the carbonation step. The main challenge is in designing an energy efficient absorber.
This first generation process technology is based on the work Ragipani et al (2022), ACS Sustainable Chemistry and Engineering.
