Bioinorganic Laboratory
Bioinorganic Laboratory
Department of Chemistry
Indian Institute of Technology Kanpur
Research Areas
We are engaged in studying a wide range of bioinorganic and biological systems, all of which fall under the general theme of gaining a better understanding of the heme centers in heme proteins that are vital to the life of almost all living organisms. A wide variety of spectroscopic techniques have been used which includes variable temperature measurements of UV-vis-NIR spectroscopy, Paramagnetic NMR, EPR, Mössbauer spectroscopy, X-Ray diffraction, Cyclic Voltammetry, Circular Dichroism, Magnetic measurements (SQUID), Fluorescence spectroscopy, XPS, etc.
Density Functional Theory (DFT) calculations are utilized to interpret the experimental results along with structure-function correlation. At present, we are engaged mainly in the following research areas:
Unfolding Mystery of Multi-Heme Proteins
|
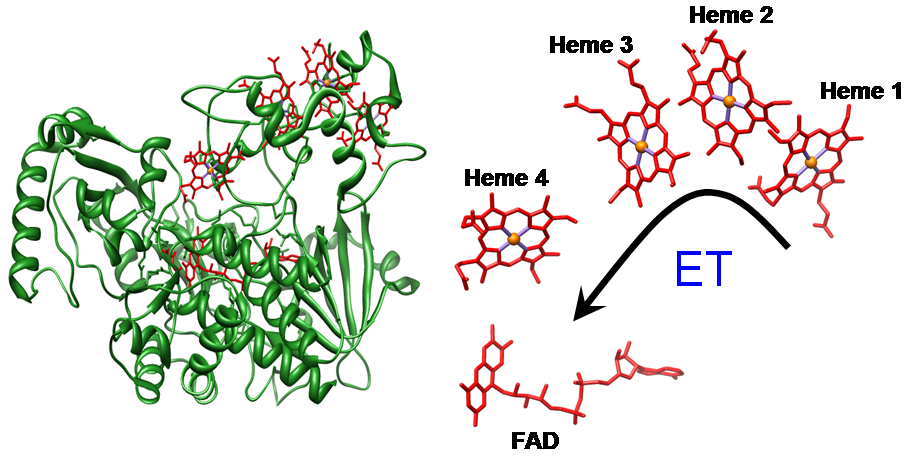
Multiheme cytochromes, that are recognizably different from the monoheme c cytochromes, constitute a widespread class of proteins with essential functions in electron transfer and enzymatic catalysis. There is a high level of conservation of heme structural arrangement throughout various multi-heme cytochromes. However, the functional significance of such arrangements is not yet understood, and they possibly reflect favorable arrangements to tune heme–heme redox potential interactions and/or warrant very fast electron transfer. Understanding the significance of these heme structural motifs is crucial for the elucidation of the highly optimized properties of multiheme cytochromes which we are currently engaged in. Interaction between heme centers has been smartly implemented by Nature in order to regulate different properties of multiheme cytochromes, thereby allowing them to perform a wide variety of functions. Our broad interest lies in unmasking the role played by heme-heme interaction in modulating different properties viz., metal spin state, redox potential etc., of the individual heme centers using ethane-bridged porphyrin dimer as a synthetic model of dihemes. The large differences in the structure and properties of the diheme complexes, as compared to the monoheme analogs, provide an unequivocal evidence of the role played by heme-heme interaction in the dihemes. For more details click here.
Representative Publications: (i) Dalton Trans. 2015, 44, 16195. (Invited Perspective Article) (ii) Dalton Trans. 2018, 47, 14388. (Invited Frontier Article)
|
|
Di-heme Enzymes: A Family with Superior Biological Activity |
|
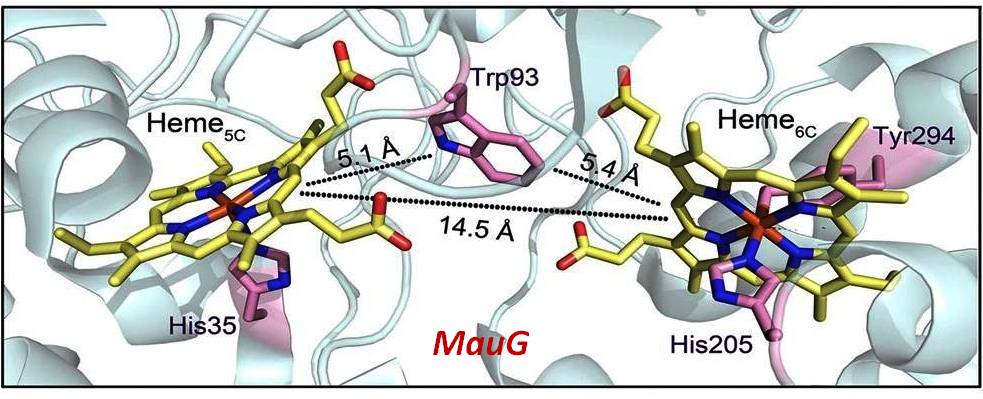
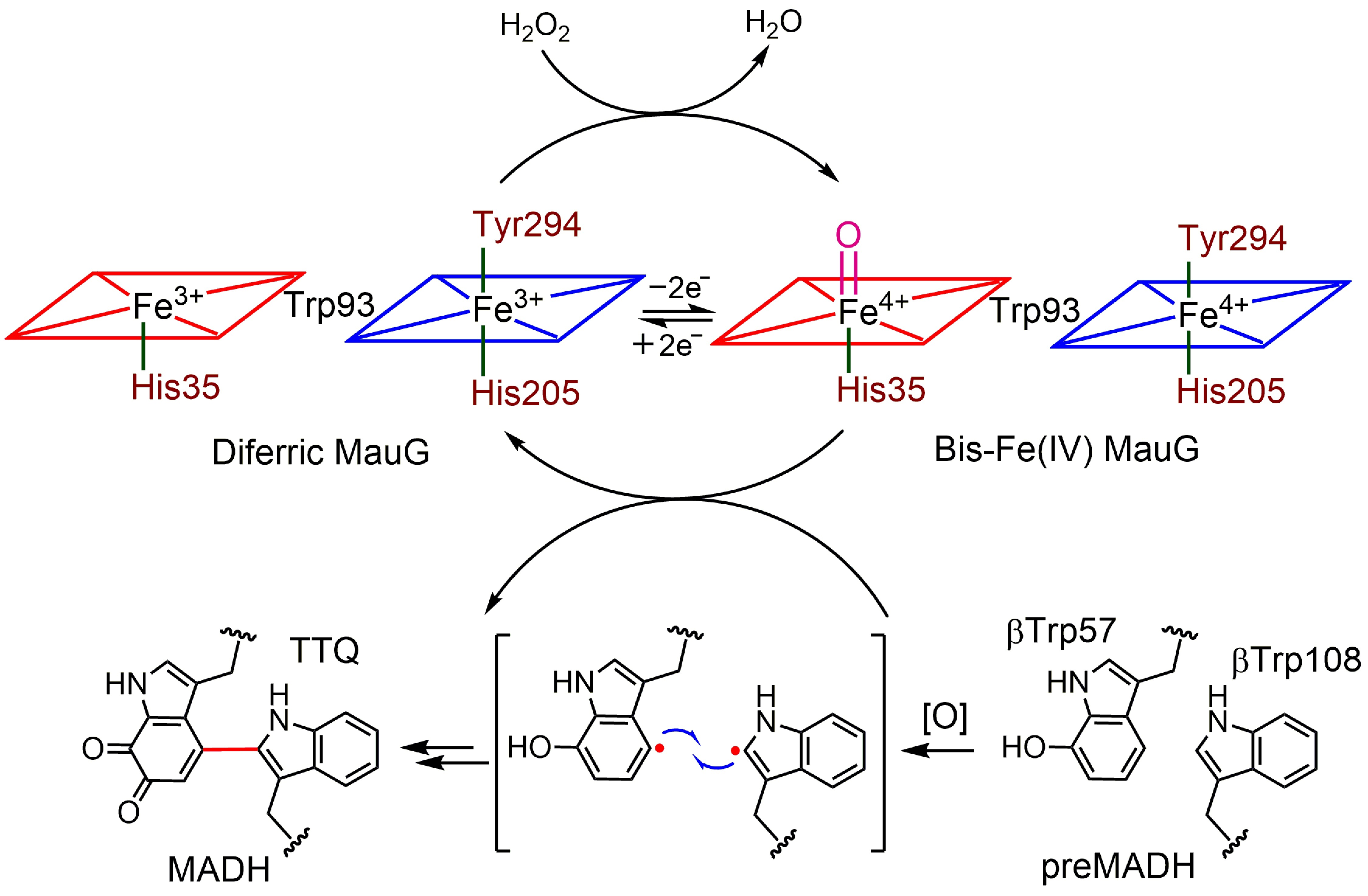
The diheme cytochrome c (DHC2) from G. sulfurreducens is the simplest member of multiheme cytochromes family, with two heme groups attached through a single polypeptide chain that are found to be different. The observed differences in the axial ligand orientations and porphyrin ring deformations between two heme centers in DHC2 have been proposed to be the consequences of heme-heme interactions although the functional significance of these heme structural arrangements is yet to be understood. MauG is a diheme enzyme that utilizes two covalently bound c-type hemes to catalyse the biosynthesis of the protein-derived cofactor tryptophan tryptophylquinone. The two hemes are physically seperated by 14.5 Å and a hole-hopping mechanism is proposed in which a tryptophan residue located between the hemes undergoes reversible oxidation and reduction to increase the effective electronic coupling element and enhance the rate of reversible electron transfer between the hemes in bis-Fe(IV) MauG. These attractive features have prompted us to investigate the relationship between such interactions and the properties of the metal center as a part of our ongoing research. For details click here.
DHC2
Representative Publications: (i) Chem. Commun. 2019, 47, 4790. (ii) Inorg. Chem. 2018, 57, 11498. (iiii) Angew. Chem., Int. Ed. 2017, 56, 8849. (iv) Chem. Eur. J. 2017, 23, 13415. (v) Chem. Eur. J. (Communication) 2017, 23, 10270. (vi) Chem. Sci. 2016, 7, 1212. (vii) Angew. Chem., Int. Ed. 2016 55, 996. (viii) Dalton Trans. 2015, 44, 16195. (ix) Chem. Commun. 2015, 51, 14107. (x) Inorg. Chem. 2014, 53, 11925. (xi) Chem. Eur. J. 2013, 19, 13732. (xii) Chem. Commun. 2011, 47, 4790. (xiv) Inorg. Chem. 2010, 49, 3449. (xv) Inorg. Chem. 2008, 47, 10196. (xvi) Inorg. Chem. 2008, 47, 9848.
|
|
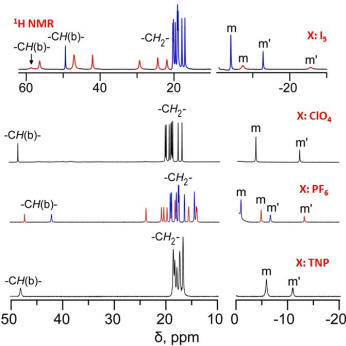
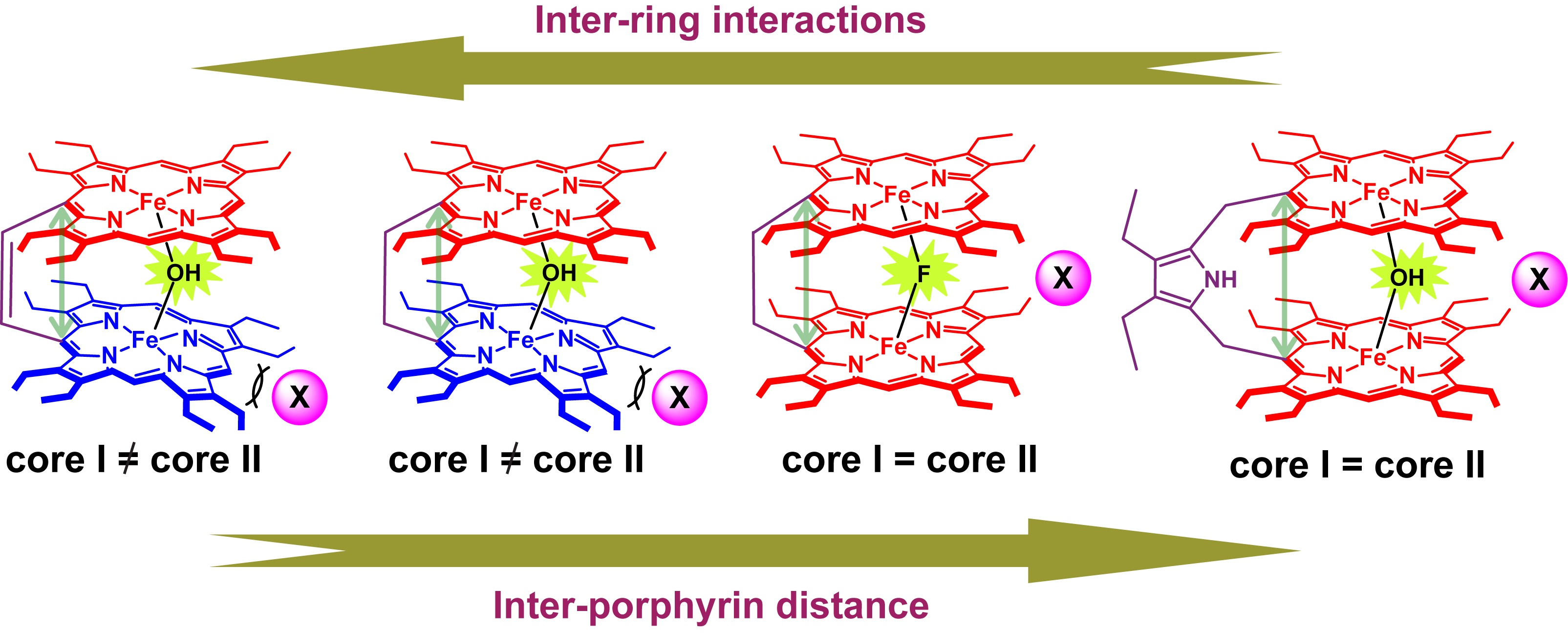
µ-Hydroxo Fe(III) Porphyrin Dimers: Modulation of Metal Spins by Counteranions |
|
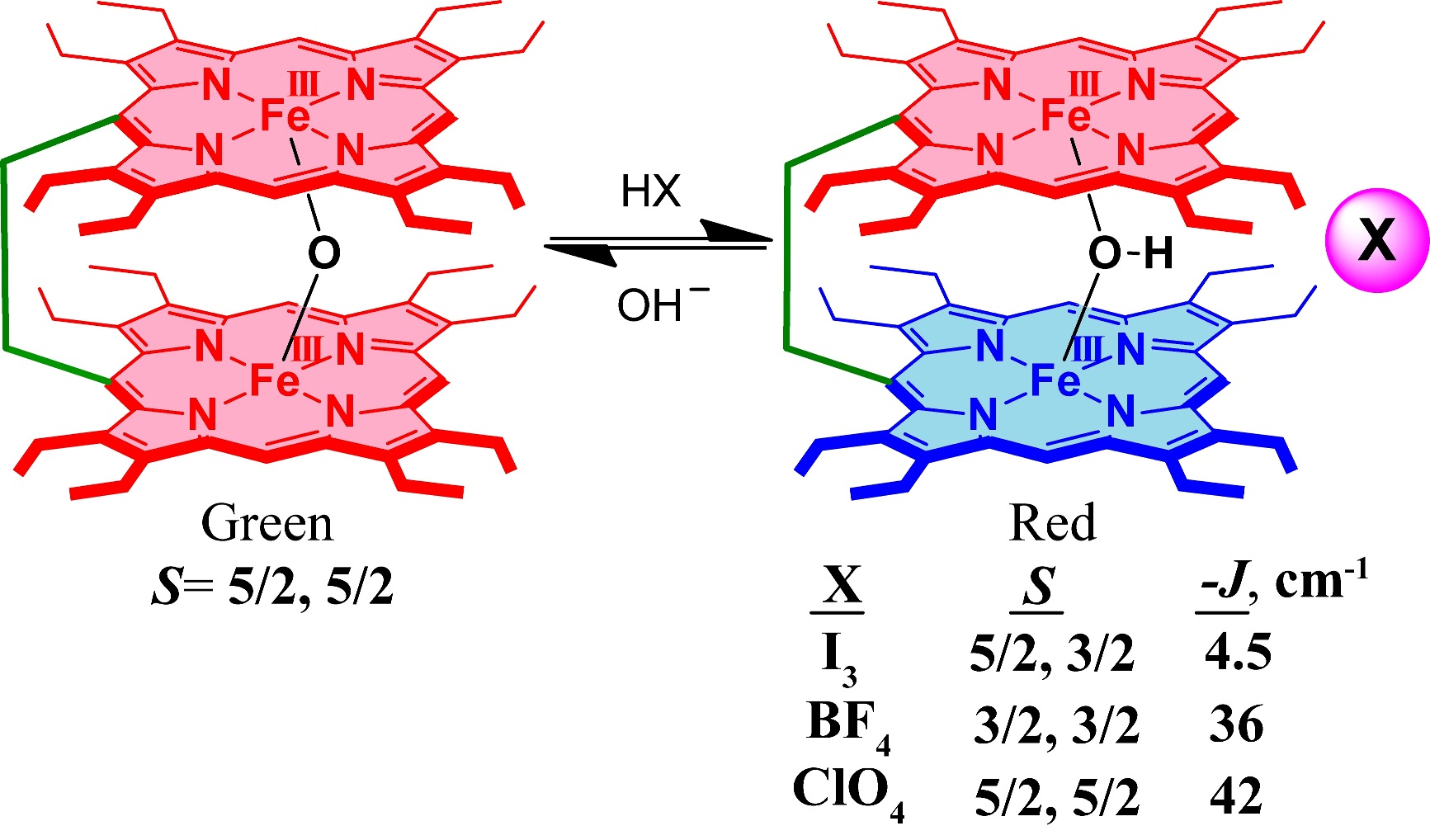
Oxo and hydroxo bridged diiron active centers are common structural motifs found in a variety of proteins in biology. The highly versatile oxo/hydroxo-bridged dimetallic centres regulated by a simple protonation/deprotonation process, permits the enzyme a natural way to control the intermediates in the catalytic cycles. The chemical and electrochemical nature of the Fe-O-Fe/Fe-O(H)-Fe unit are suitably controlled by the inter-macrocyclic interaction between two heme centers and thereby influence the structure, property and reactivity of the molecule to a large extent. A close approach of the two rings in the ethene and ethane-bridged μ-hydroxo complex results in an unequal core deformations that leads to unusual stabilization of two different spin states of iron(III) in a single molecular framework. Also, spin states vary on the counter ion (X) used and are reversibly interconvertible although the counter anions are far away and not apparently involved in any kind of direct interactions with the metal ion. The small environmental perturbations coming out of the counter anion, H-bonding interaction, heme-heme interaction etc. can change the structure and properties of the individual heme centers drastically and it seems very likely that Nature uses some of these techniques to control the property of the biomolecules for a specific function. For details click here.
Representative Publications: (i) Coord. Chem. Rev. 2017, 334, 112. (ii) Dalton Trans. 2017, 46, 1012. (iii) Chem. Eur. J. 2016, 22, 16124. (iv) Chem. Eur. J. 2016, 22, 14585. (v) Chem. Eur. J. 2016, 22, 11214. (vi) Inorg. Chem. 2015, 54, 1919. (vii) Chem. Eur. J. 2013, 19, 17846. (viii) Chem. Eur. J. 2013, 19, 13732. (ix) Chem. Eur. J. 2012, 18, 13025. (x) Chem. Commun. 2011, 47, 4790. (xi) J. Am. Chem. Soc. 2010, 132, 17983. (xii) Inorg. Chem. 2010, 49, 3449. (xiii) Inorg. Chem. 2008, 47, 10196. (xiv) Inorg. Chem. 2008, 47, 9848. |
|
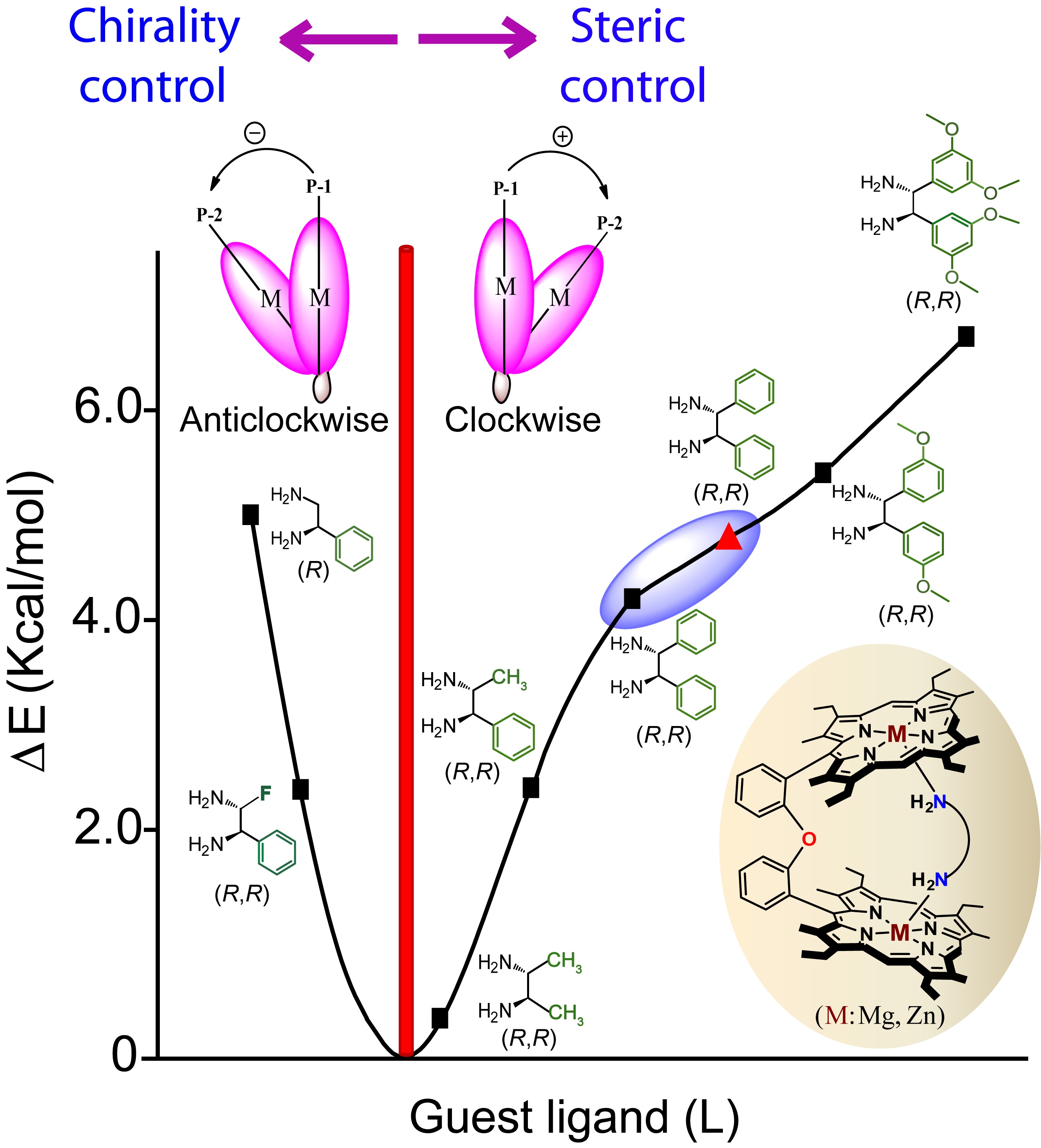
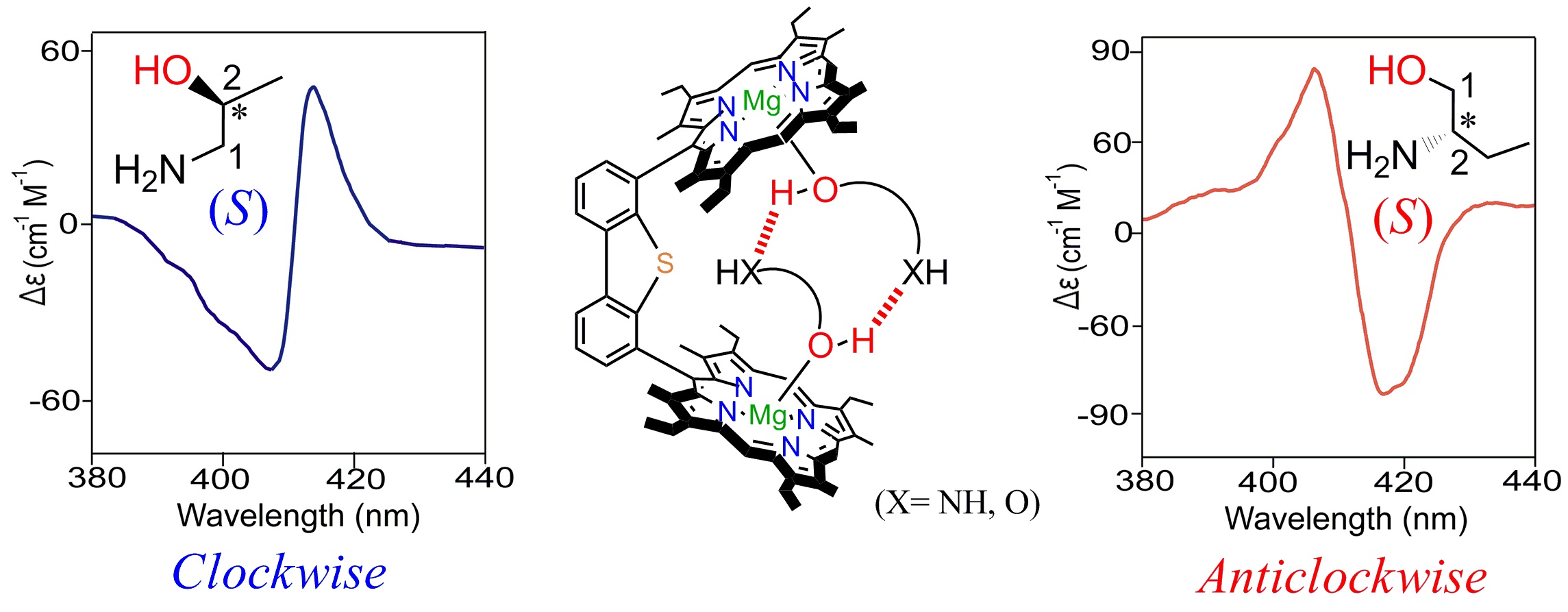
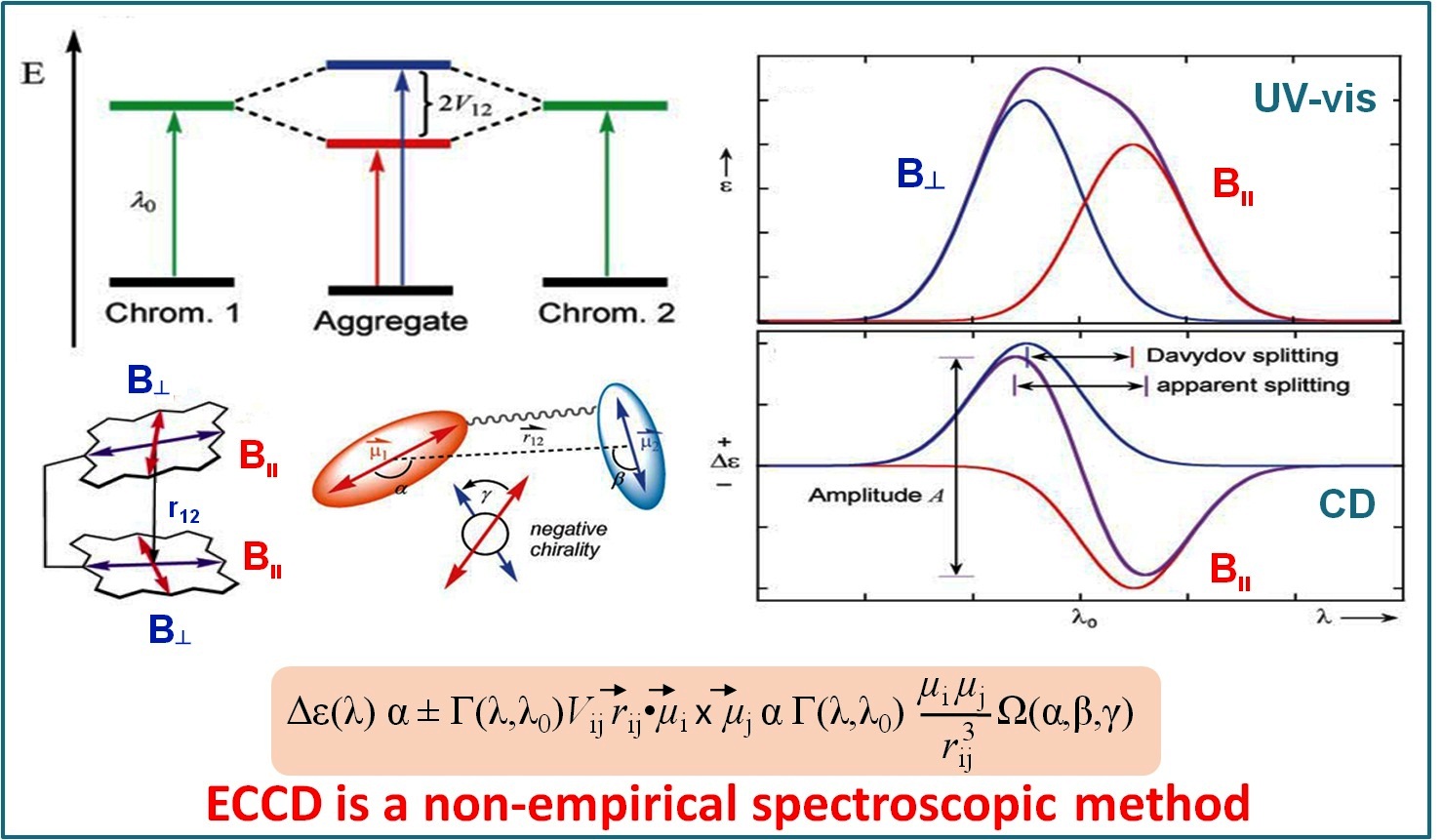
Probing Molecular Chirality using Metallo-Bisporphyrin Hosts |
|
Supramolecular chirogenesis is one of the most important interdisciplinary field to be looked into, because of its occurrences in many natural (DNA double helix, heme proteins, secondary α-helix structure of proteins etc.) and artificial systems. Exciton Coupled Circular Dichroism (ECCD) is a nonempirical spectroscopic method that is based on detecting the through-space exciton interaction between helically orientated independently conjugated chromophores. The challenge lies in orienting two or more chromophoric receptor groups in a chiral fashion as a direct result of the binding of a chiral compound and extrapolating the chirality of the bound compound from the ECCD spectra. Our group is currently engaged investigating the various aspects of chirality induction and control for probing molecular chirality using ECCD. For details click here.
Representative Publications: (i) Inorg. Chem. 2019, 58, 0000. (ii) Inorg. Chem. 2017, 56, 15203-15215. (iii) Inorg. Chem. 2017, 56, 3849. (iv) Inorg. Chem. 2016, 55, 13014. (v) J. Org. Chem. 2016, 81, 5440. (vi) Chem. Commun. 2015, 51, 895. (vii) Chem. Commun. 2015, 51, 14107. (viii) Chem. Commun. 2014, 50, 14037. (ix) Inorg. Chem. 2014, 53, 49. (x) Inorg. Chem. 2014, 53, 2381. (xi) Chem. Commun. 2012, 48, 4070.
|
|
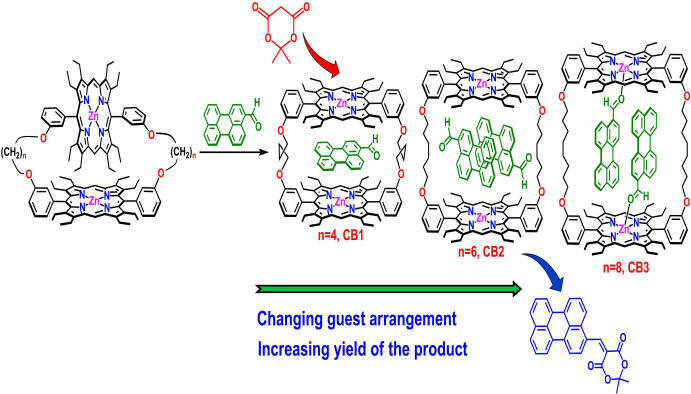
Artificial Cavity for Enzymatic Reactions |
|
Simple synthetic receptors with molecular pockets or cavities can act as model compounds for many complicated biological systems. The development of synthetic host that are capable of binding substrates that mimic various chemical processes in nature has long been an important goals. Covalently linked porphyrin dimeric architectures have been attracting considerable attraction originally based on their involvement in photosynthetic events, but they have also taken a new turn towards various potential applications. For details click here.
Representative Publications:
(i)
Eur. J. Inorg. Chem. 2019, 0000.
(ii)
Chem. Asian J.
2017, 12,
1824.
(iii)
Chem. Eur. J.
2017,
23, 7093. (iv)
Chem. Eur. J.
2016, 22, 5607.
(v)
Eur. J. Inorg. Chem.
2015, 4956.
(vi)
Dalton Trans.
2014, 43, 2301.
(vii)
Inorg.
Chem.
2012, 51, 9666.
(viii)
Dalton Trans.
2013, 42, 12381.
(ix)
Chem. Eur. J.
2012, 18, 7404.
(x)
Chem.
Eur. J.
2011, 17, 11478.
|
|
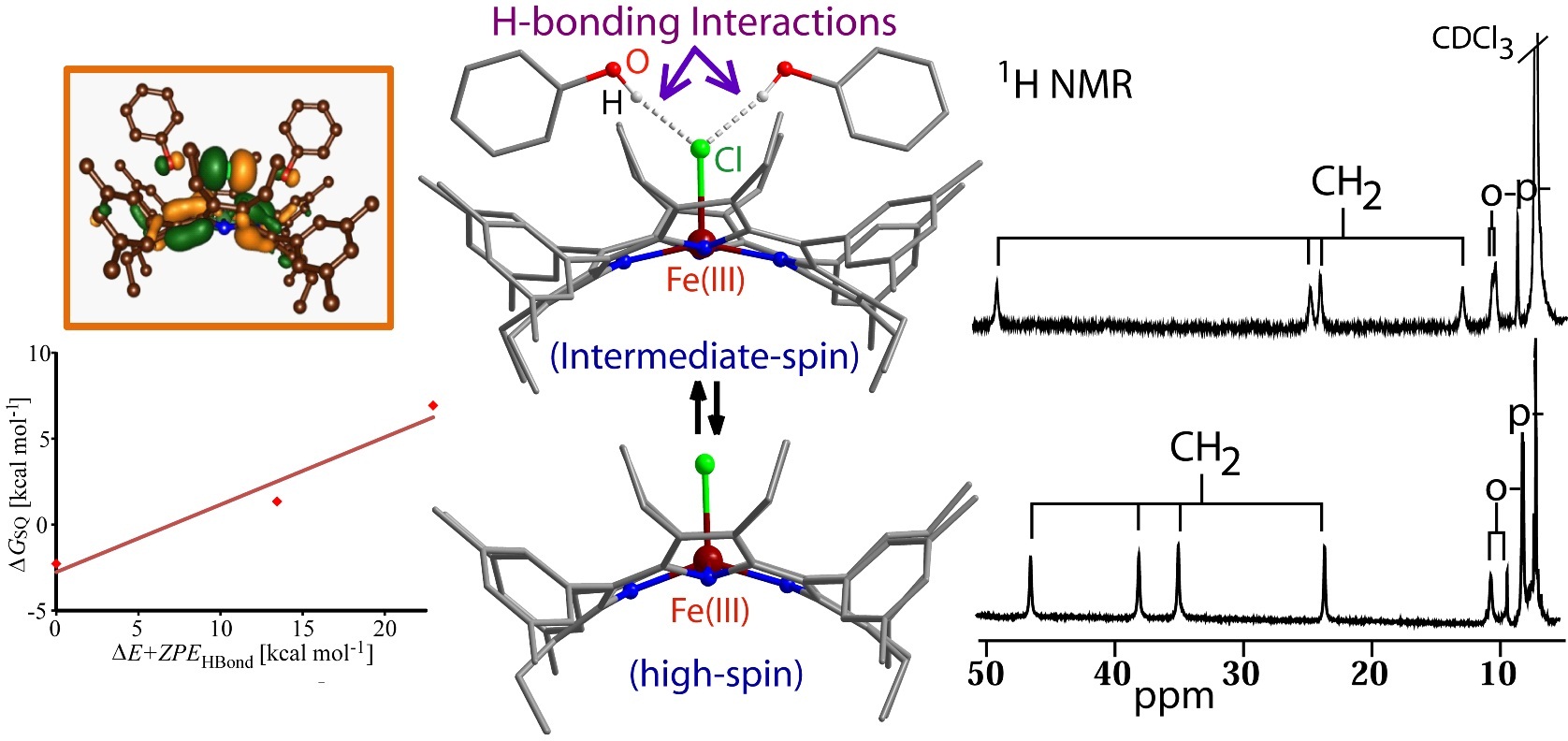
Mono-Heme Cytochromes: Structure-Function Correlation |
|
Hemoproteins serve many diverse biological functions through the nearly identical heme prosthetic group that displays a range of distorted nonplanar shapes. A key step in cytochrome P450 catalysis includes the spin-state crossing from low-spin to high-spin upon substrate binding and the subsequent reduction of the heme. To gain insight, we have studied the impact of H-bonding interactions on the electronic structure of a five-coordinate iron(III)octaethyltetraarylporphyrin chloride. The nature and extent of non-planarity in heme was successfully demonstrated to be instrumental in modulating the metal ion displacement from porphyrin mean plane, axial ligand orientations, metal spin and redox properties and even axial ligand affinity. For details click here.
Representative Publications: (i) Eur. J. Inorg. Chem. 2016, 3441. (ii) Eur. J. Inorg. Chem. 2016, 3305. (iii) Angew. Chem. Int. Ed. 2015, 54, 4796. (iv) Chem. Commun. 2015, 51, 16790. (v) Inorg. Chem. 2012, 51, 11294. (vi) Eur. J. Inorg. Chem. 2010, 10, 5211. (vii) Dalton Trans. 2010, 39, 5795. (viii) Inorg. Chem. 2010, 49, 2057. (ix) Inorg. Chem. 2008, 47, 8324. |
| 2021 |
.jpg)