| |
Ideal Gas
The kinetic theory of gases model a gas as a collection of elastic particles. The statistical analysis of these with some assumptions results as the ideal gas law
- Most gases deviate from ideal gas in the vicinity of the critical point and saturated vapour line
.
- Most gases behaves like ideal gas at high temperature and low pressure regions.
Joules Experiment Refer to figure 9.1
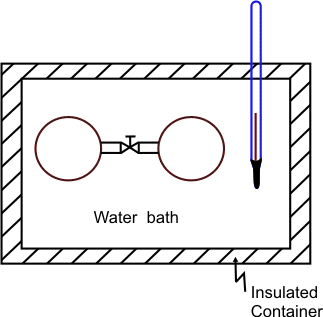
Figure 9.1
One of the vessels was filled in with air at 22 atm pressure and the other vessel was evacuated. The initial temperature of the water bath was measured. The valve was opened allowing air to occupy both the containers. The temperature of the water bath was measured again and it was found that the temperature did not change. The gas contained in both the vessels is our system. W=0 and Q=0. Since the number of moles of the gas also remains constant, it follows that 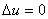 . The pressure and the volume of the gas changed and yet the internal energy did not change. Hence . The pressure and the volume of the gas changed and yet the internal energy did not change. Hence
So,
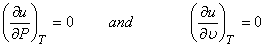 |
(9.1) |
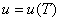 |
(9.2) |
Since,
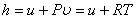 |
(9.3) |
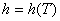 |
(9.4) |
|