Research Areas
Solid Oxide Fuel Cells (SOFCs)
The technology based on solid oxide fuel cells (SOFCs) is demonstrated to be the most efficient in generating clean energy. This solid-state electrochemical device with no moving parts can show efficiency > 80% (with cogeneration) in converting the chemical energy of the fuels and oxidants to electrical energy. Owing to its high-temperature operation (~1000°C), it can also run on a wide range of fuels such as H2, syngas, biogas, natural gas, CO, CO2, landfill waste, etc. However, this also means requiring expensive and specialized component materials to withstand the stringent environment for a long duration. It has been realized that the successful adoption of SOFC technology, especially for stationary and residential electrical power generation, is only possible when the operating temperature is lowered to an intermediate temperature range (500-850°C) without reducing the performance output. This necessitates the development of innovative materials with improved functionalities and more affordable costs.
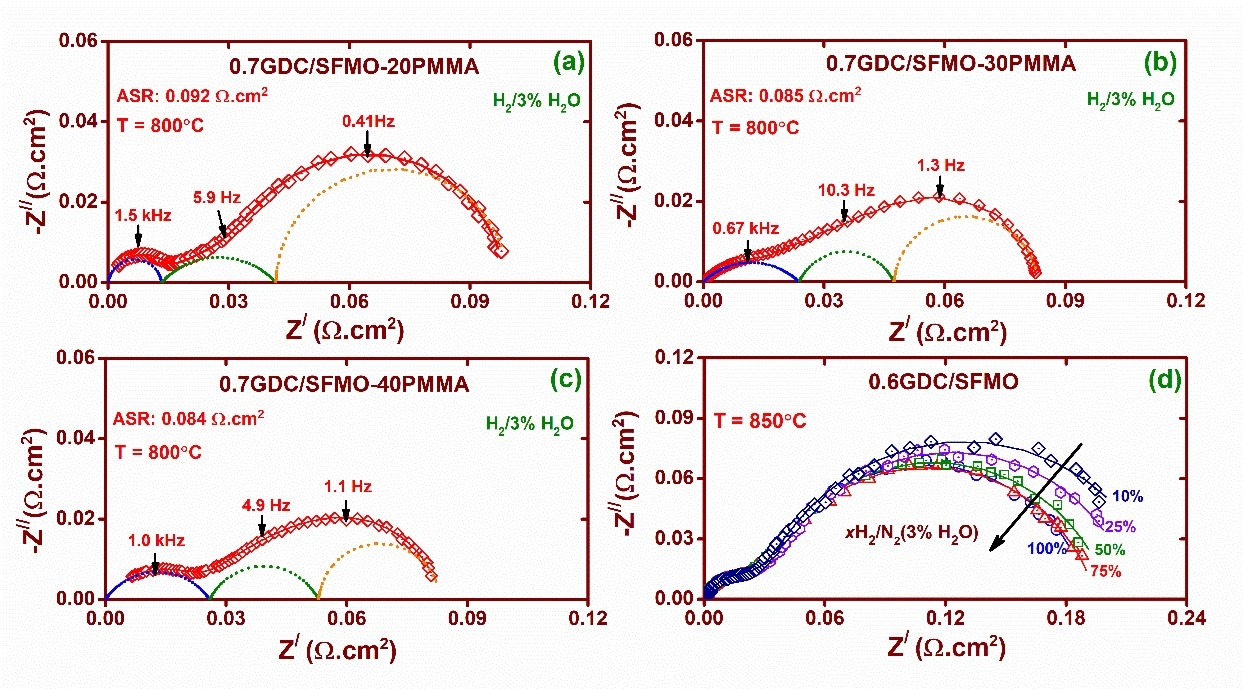
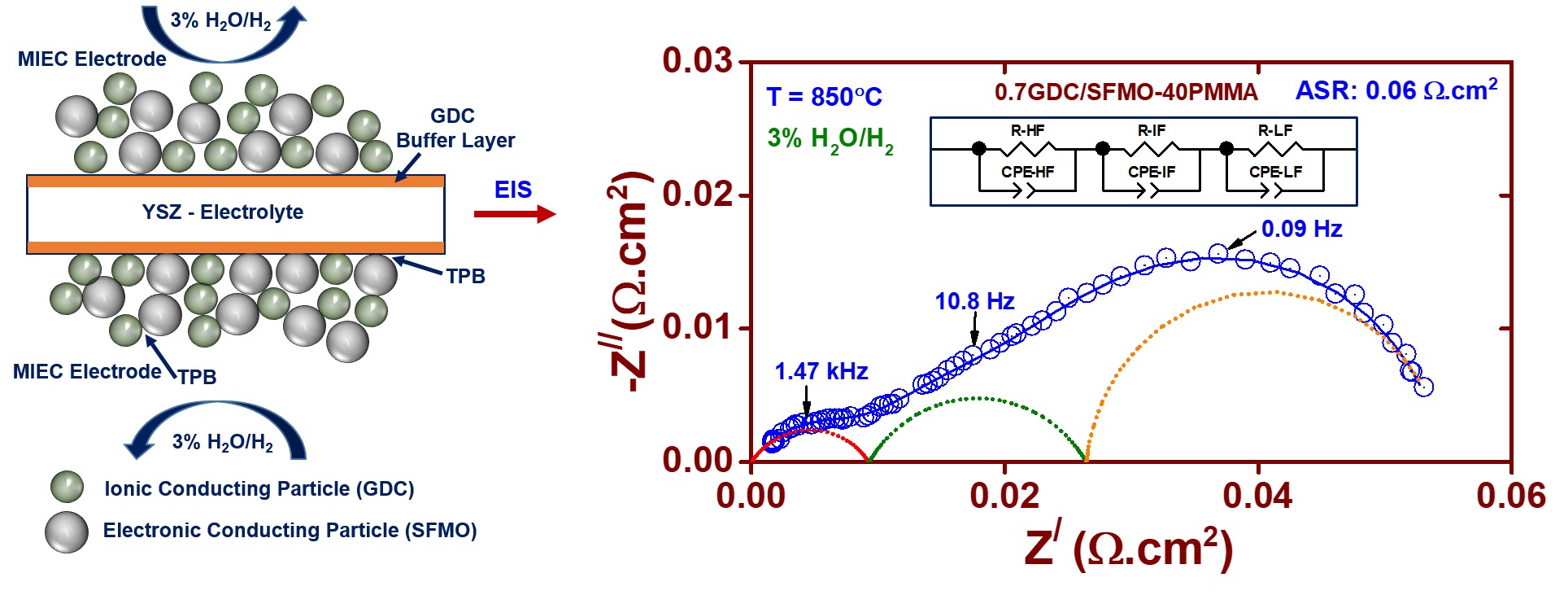
Our research group focuses on the development of materials for solid electrolyte and electrodes. For the electrolyte, we characterize the ionic conductivity and study its correlation with the structure, chemistry and processing parameters of several oxygen-ion conductors based on CeO2, ZrO2, NBT, etc. We also investigate the ASR behaviour of composites based on SrMoO3 and SrFeO3 for the electrode application in SOFCs. Efforts are made to fabricate the prototype of SOFCs using the novel materials and test them in a typical operating condition.
Battery
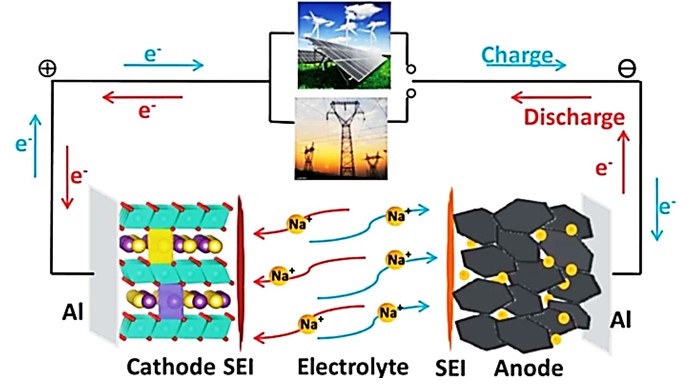
Rechargeable batteries convert chemical energy into electric energy and vice-versa through reversible faradaic redox reactions. Initially, Li-ion batteries (LIBs) became hugely popular because of their efficient performance and higher rate capabilities. LIBs currently power most of the consumer electronics in the market. However, the high cost and limited availability of Li mineral resources have driven research to look into other alternatives. Na-ion batteries (NIBs) are considered next-generation batteries as they promise to become a low-cost storage option for future electric grids as intercalation chemistry in sodium and lithium are similar. Besides, the Na element is the sixth most abundant element on earth crust and is readily available.
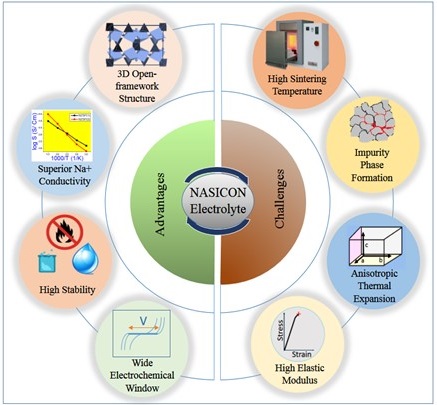
Our research group focuses on designing materials for all-solid-state NIBs. All-solid-state NIBs possesses solid electrolyte and thus addresses the serious safety issues associated with liquid organic electrolytes. We study the process-structure-property correlations in doped NASICON-type oxides necessary to rationally design the high sodium-ion conductive materials for solid electrolyte applications. Further, our group also investigate oxide-based cathodes for NIBs. Our research laboratory has the facility to fabricate and electrochemically test the performance of coin cells for batteries application.
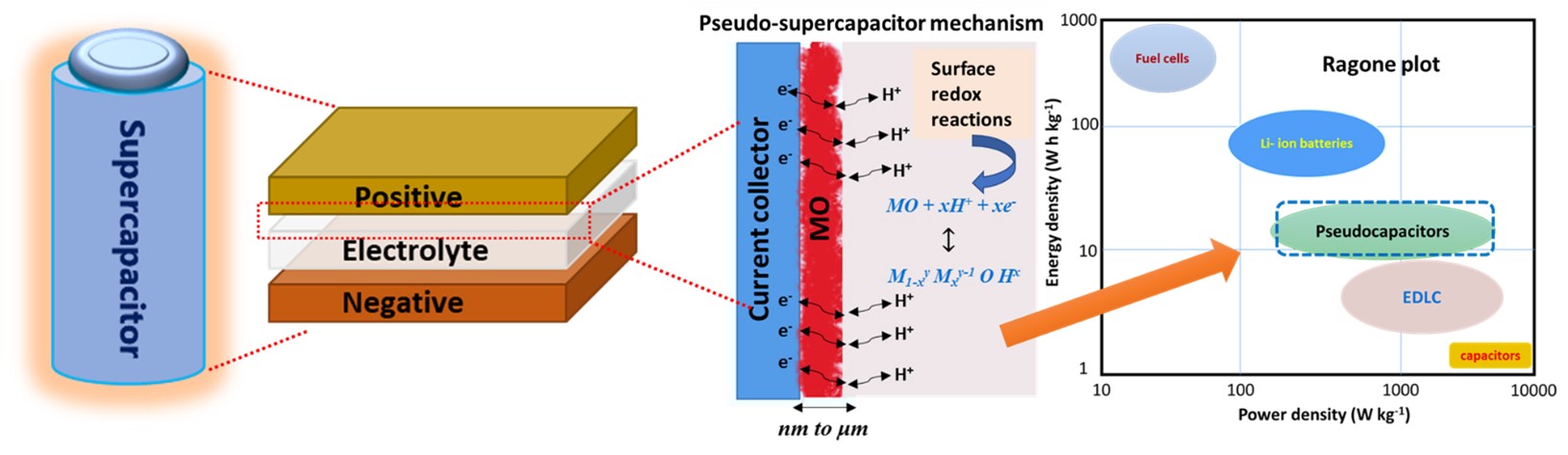
Supercapacitors
Supercapacitors (SCs) are considered as a complimentary energy storage system to batteries, which exhibit superior energy density than conventional capacitors and higher power density than batteries. The long cycle life of SCs has driven their usage in automobiles, electric locomotives, smart phones, etc. To date, carbon based double layer SCs are commercialized displaying high power density, however, their limited energy density remains a constraint. Later, pseudocapacitors came into picture involving redox reactions driven charge storage mechanism. The mechanism provides substantial increment in the energy density of the order of ~10 or more. Our group's research focuses on the development of novel strategies for the fabrication of advanced supercapacitors. The work involves:
- Synthesizing new electrode materials (oxides/chalcogenides/phosphides) with intriguing high surface area morphologies
- Stabilization of freestanding binder-free electrodes
- Electrolyte optimization for enhanced voltage window using redox additives
- Device performance optimization at ambient and non-ambient conditions by synergistic correlation of electrodes and electrolytes redox chemistry
H2 production
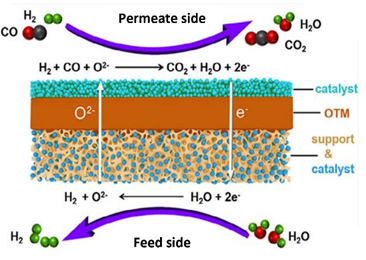
MIEC membrane allows conduction of oxygen ions and electrons through the means of oxygen vacancies present inside the lattice structure (as shown in fig). These vacancies are created due to non-stochiometric nature of material at high temperatures (600-800oC). As these vacancies can only be filled by oxygen ions, MIEC membrane ideally have 100% selectivity for oxygen. This characteristic can be used to obtain high purity hydrogen as shown in fig, it shows a schematic of a membrane reactor where syngas, a product obtained from gasification or partial oxidation of methane, is passed through the permeate side of the membrane, while steam is passed through the feed side. Steam on the feed side reduces to H2, while O2- permeates through the membrane to the permeate side, where it oxidizes syngas, producing a mixture of CO2 and water vapor. Through this very high-purity H2 can be produced.