Research
Biophysical Chemistry
Solution Chemistry
Molecular-level structure and dynamics decide the functionality of a solvent. Therefore, a significant amount of effort was dedicated, being dedicated, and will be dedicated in understanding its structural and dynamical features. Using various steady-state and time-resolved fluorescence measurements and NMR spectroscopy, we try to understand the following
Current research:
- We are aiming for better molecular-level picture of the synergistic solvation in a binary solvent mixture and DES
- Structure and dynamics of the warm-like micelle.
- Understand various forces and interactions responsible for the unique behaviour of binary mixture and DES.
- Synergism in DES and hydrated DES.
Contact:
Synergism solvation in the binary solvent mixture
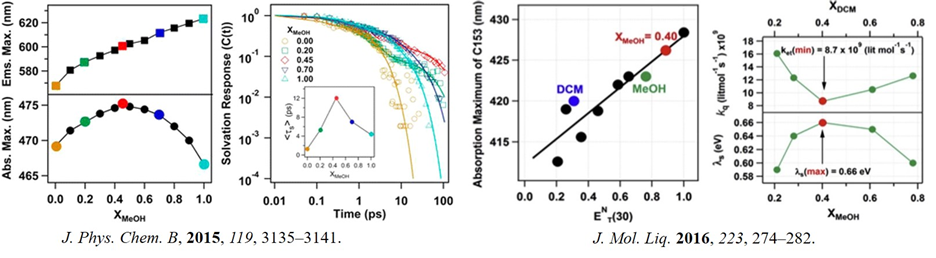
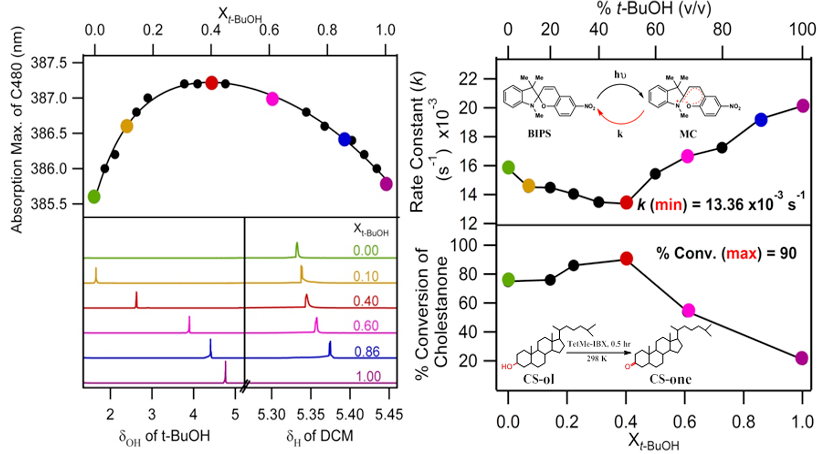
The non-ideality in the binary solvent mixture is manifested through anomaly in various physical properties like viscosity, dielectric constant, polarity, freezing point, boiling point and so forth. Sometimes, such anomalies become useful in chemistry and biology. From a physical chemist's perspective, understanding such a novel feature is fascinating to us. The source of non-ideality in a binary solvent mixture can be broadly categorized in two cases: preferential and synergistic solvation. The synergistic effect in various primary alcohols/chlorinated methane has been thoroughly studied by our group and is attributed to unique hydrogen bonding. Using 1H-NMR spectroscopy, we found that methanol and chloroform form a 1:2 H-bonded complex between them, which possess a more polar environment than the individual solvents (J. Phys. Chem. B 2012, 116, 1345; Phys. Chem. Liq. 2018, 56, 496). The solvation time was found to be maximum in XMeOH = 0.45 in the same system (J. Phys. Chem. B, 2015, 119, 3135). Similar type of synergism was observed for ethanol/chloroform and t-butanol/chloroform solvent mixtures (J. Mol. Liq. 2016, 223, 274; J. Sol. Chem. 2017, 46, 461). Interestingly, carbon tetrachloride, which does not have any donor hydrogen, does not show synergism with primary alcohols. Finally, the t-butanol/dichloromethane binary solvent mixture was shown to affect the yield of a chemical reaction's product due to synergistic solvation.
Understanding the confined solvent system
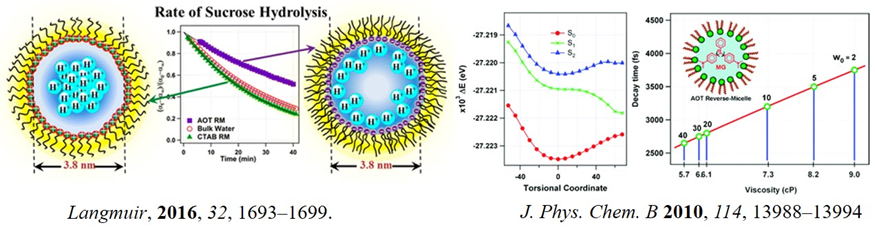
The behaviour of a molecule drastically changes under confinement. Therefore, investigating solvent in the confined environment might have implications on the behaviour of biological systems. Using femtosecond fluorescent up-conversion measurement of malachite green, we probe the micro-viscosity of water trapped in a nanoconfined environment using an AOT reverse micelle. The data confer that the microviscosity of water in an water pool of W0=2 is almost nine times higher compared to bulk water. As W0 is increased, microviscosity decreased monotonically (J. Phys. Chem. B 2010, 114, 13988). In another project, we addressed the "acidity" of the water pool of AOT reverse micelle. We propose that the buffer-like action in the water pool is much more decisive than expected earlier. It has been concluded that a proton gradient exists inside the water pool of reverse micelle and it determines the buffer-like action of the water pool that persists until about 2N of HCl in AOT of W0=10 (Langmuir 2016, 32, 1693).
Molecular-level understanding of deep eutectic solvent (DES)
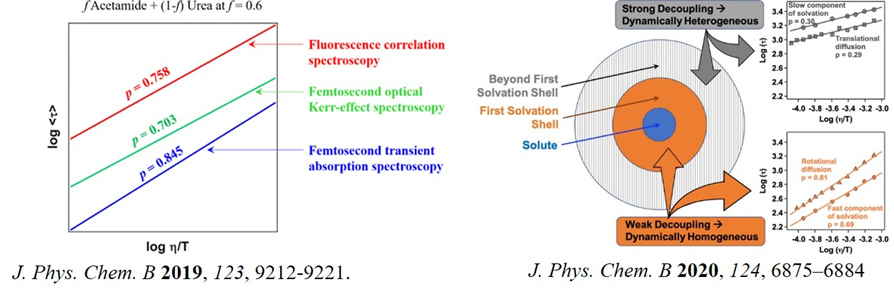
Deep eutectic solvents (DES) are emerging as a new class of media for many practical applications, including electrochemistry, biocatalysts and extraction due to their environmentally friendly nature, fine-tunability, and potential bio-compatibility. Recently, we took up the project to understand the molecular level structure and dynamics of such solvents. We studied temperature-dependent translational diffusion and solvation dynamics of non-ionic urea/acetamide DES to prove that the system is moderately heterogeneous (J. Phys. Chem. B 2019, 123, 9212). A similar study to DES composed of acetamide and various metal nitrate salts proves the system to be highly dynamically heterogenous (J. Phys. Chem. B 2020, 124, 1995). Such heterogeneity might have implications in understanding the variety of impacts of DES. The main barrier of DES to be used in practical application is its high viscosity. We, therefore, move to a DES comparatively less viscous: lauric acid menthol. Our result suggests that the system is spatially (structurally) homogenous, but dynamically heterogenous. Interestingly, we proved that extent of dynamic heterogeneity depends on the length scale (J. Phys. Chem. B 2020, 124, 6875).

